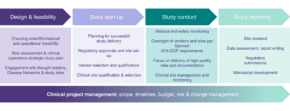
Ensuring robust planning and oversight are critical success factors to deliver clinical trials. Sponsors of clinical trials are accountable for ensuring a study is conducted in accordance with the protocol, GCP, and applicable regulatory requirements.
tranScrip provides customised solutions to support delivery of our client’s clinical studies. We build lasting relationships with our clients that go above and beyond the typical service provider transactional relationships. We achieve this through our collective industry experience, substantial networks, and know-how. We work as a seamless team with our clients, sharing development objectives by providing continuity with project teams and going above and beyond.
A successful clinical study is conducted in accordance with the protocol, with ICH-GCP and with delivery of high-quality data. Sponsors of clinical studies are accountable for ensuring this and need to assign adequate resource for effective planning, risk management, vendor oversight and quality assurance.
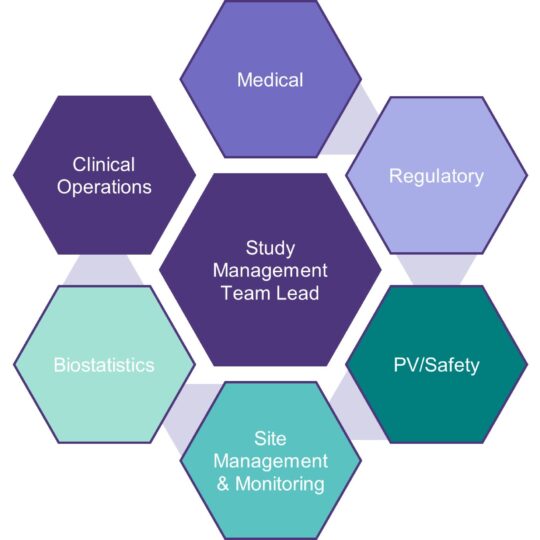
Ideally, a Study Management Team is established early on during the study design phase. This enables an early and thorough assessment of opportunities and risks. Smart planning, with a focus on critical data and process, significantly decreases the risks associated with data quality and study delays, both of which can be costly.
Outsourcing clinical studies is a significant investment and it is critically important to choose the right solution for you. Having the right expertise to hand when you need it, is an advantage.
tranScrip provides a customised and flexible approach which enables us to provide support from individual Subject Matter Experts (such as medical monitors, study managers) to whole cross-functional Study Management Teams.
Our clients don’t just have access to the assigned project team but also rapid access to the wealth and breadth of the wider tranScrip team of experts and extensive network when needed.
We work “Sponsor-side” as an extension of your team, sharing your development objectives. We have a strong ethic of ensuring our clients make data-driven, smart decisions. We want our clients to be successful and will treat your programme as our own.
We have teams of life science and R&D experts to help achieve your goals and seize opportunities. Whatever your project requires, we have the people to meet your needs.